Introduction: Light chain Amyloidosis (AL) is a systemic disease caused by abnormal clonal plasma cells which produce excessive light chains that further misfold and deposit into vital organs. Currently, treatment options for AL amyloidosis are relatively limited. Daratumumab (dara), a novel monoclonal antibody that targets CD38 on the surface of plasma cells, has shown remarkable efficacy in AL amyloidosis. Our objective was to explore the pattern of use, safety and efficacy of dara therapy among AL amyloid patients in a real-world setting at a single institution.
Methods: We performed a retrospective analysis of patients with AL amyloidosis diagnosed by tissue biopsy who received dara-based therapy from 11/16/2015 to 3/16/2020 at Tufts Medical Center. The study was approved by the Tufts Medical Center Institutional Review Board. Baseline demographics, clinical characteristics and therapy-related data from patients were extracted from the electronic medical records. Kaplan-Meier method was used to estimate time to hematologic response, time to organ response and progression free survival (PFS) after dara initiation. Log-rank tests were applied to compare PFS among subgroups and to explore the impact of involved free light chain (iFLC) levels on organ response. Results were considered to be significant if two-sided P-value was less than or equal to 0.05. R software was used for statistics.
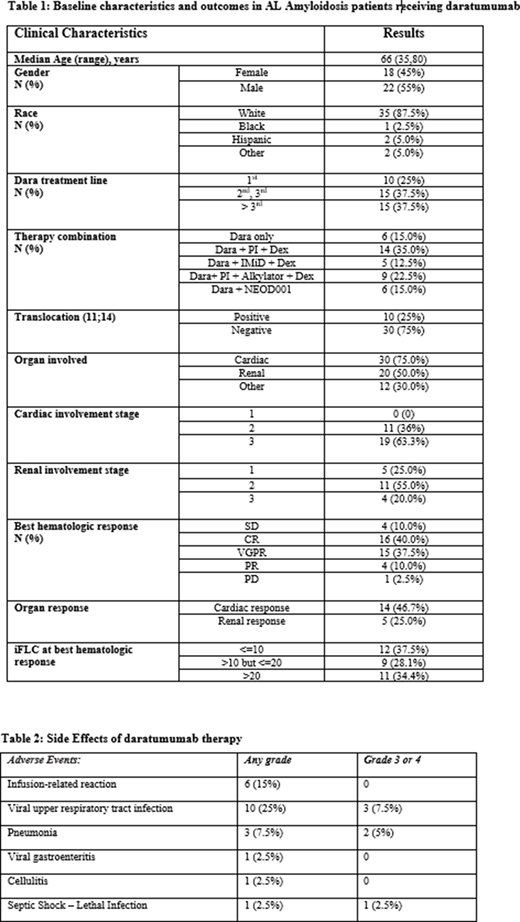
Results: Forty AL patients were included in the study with a median age of 66 years, ranging from 35 to 80 years. Among these patients, 22 (55%) were male and 35 (87.5%) were non-Hispanic White. Cardiac involvement was present in 30 subjects (75%) and renal involvement was present in 20 (50%). By cytogenetic stratification, 10 (25%) individuals had translocation (11;14). Table 1 highlights the clinical characteristics of the study population including the combinations of dara with traditional therapies. Dara was well tolerated in the majority of cases with only one instance of discontinuation due to frequent upper respiratory tract infections. The major therapy-related adverse events are highlighted in table 2. The overall hematologic response rate was 87.5%, including 16 complete responses (CR), 15 very good partial responses (VGPR), and 4 partial responses (PR). The estimated median time to hematologic response was 1.5 months (95% confidence interval (1.0, 2.0 months). The overall cardiac response rate after dara treatment was 46.7%, and the estimated median time to cardiac response was 8.25 months (95% CI, 5.5, 13.0 months). Likewise, the overall renal response rate was 25.0% with the estimated median time to renal response being not evaluable (NE) (95% CI, 12.0, NE). There were 31 patients who had hematologic progression through the study period; the median PFS was 12.0 months (95% CI, 8.25, 18.50 months). The median PFS among those who had translocation (11;14) was 24.1 months. Further log-ranks tests showed that there was no significant difference in PFS when subgrouping patients based on line of treatment (1st vs. 2nd/3rd vs. >3rd, P = 0.322) or pattern of therapy (dara monotherapy vs. combination with PI vs. IMiD vs. PI + alkylating agents vs. NEOD001, P = 0.151). Among the subjects who achieved at least a VGPR, iFLC levels (<10 vs. 10-20 vs. >20) did not show significant impact on the rate and time to organ response (cardiac, P = 0.551 or renal, P = 0.260). In the same population, it was also found that line of treatment (1st vs. 2nd/3rd vs. >3rd) was not associated with organ response (cardiac, P=0.950 or renal, P=0.817).
Conclusions: Among the AL amyloidosis patients at Tufts Medical Center treated with dara over the last 5 years, the overall hematologic response was high (87.5%) and the median time to response was rapid (1.5 months), consistent with findings from prior trials. Most importantly, dara was well-tolerated. Patients with t(11;14) had a mPFS that was double the rest of the population (24 vs. 12 months) These results are promising for the disseminated use of dara in AL given its good tolerability and rapid onset of action, regardless of line of therapy. This is especially important in a disease where time is a critical factor in organ recovery.
Comenzo:Takeda: Consultancy, Research Funding; Caleum: Consultancy; Sanofi: Consultancy; Unum: Consultancy; Prothena: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Amgen: Consultancy; Karyopharm: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.